Abstract
Introduction: As patients (pts) diagnosed with chronic myeloid leukaemia (CML) in CP are predicted to have a life expectancy comparable to that of the normal population, clinical concern has focused on the burden of long-term side effects and quality of life. Stopping tyrosine kinase inhibitor (TKI) is possible in a selected group of pts, resulting in a 50% chance of treatment free remission (TFR). Limited data, however, are available on the outcome of TKI dose reduction (DR) in maintaining molecular responses.
Methods: We retrospectively analysed the outcome of TKI DR in pts in ≥MR3 treated at our centre from Jan 2000 until May 2015. We defined different low dose groups (LDG), according to the actual TKI dose: for imatinib (IM), 300mg and 200mg; for dasatinib (DAS), 70-80mg, 50mg, 40mg and 20mg; for nilotinib (NIL), 400-450mg, 300mg or ≤200mg; and for bosutinib (BOS), 300mg, 200mg and <200mg.
Given the 'real life' setting, pts may have received either 1) multiple DRs of the same TKI or 2) different TKIs at different low doses. For scenario 1): we analysed the dose that maintained ≥MR3 and was used for the longest period of time. In case of loss of molecular response on a lower dose level, the next dose was considered a further 'case' and the patient was analysed more than once. In scenario 2) the patient was analysed once for each TKI received at low dose. MR3 and MR4 were defined conventionally. The molecular recurrence free survival (MRFS) was estimated by Kaplan-Meier.
Results: We included 232 pts (IM=83 pts, cases=85; DAS=75, cases=79; NIL=72, cases=73; BOS=32, cases=33), of whom 8 pts were included in two different LDG on the same TKI (because of loss of response on the lower dose: imatinib n=2, dasatinib n=4, nilotinib n=1, bosutinib n=1). 22 and 4 pts received 2 low dose (LD) TKIs and 3 LD TKIs respectively. The total number of cases was 270. Reasons for DR included any degree of adverse event deemed significant by the clinician or pre-emptive DR at the time of introduction of a subsequent TKI due to intolerance to the previous TKI. All IM pts were treated first line, whereas the majority of pts (n=159, 88.8%) received their current 2GTKI as ≥ 2 line. Median follow-up on LD TKI was 25.3 months (1.9-175). Patient characteristics by TKI are shown in Tables 1-4.
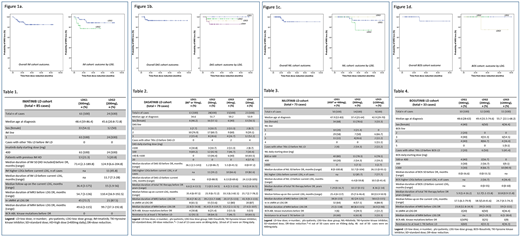
The 2-year MRFS were: IM, 88.4% (95% CI, 87.7-89.1%), and 92.7% and 77.2% for LDG1 and LDG2 respectively; DAS 92.8% (95% CI, 92.2-93.4%); and 100%, 96.2%, 92.3% and 85.6% for LDG1, LDG2, LDG3 and LDG4 respectively; NIL 93.4% (95% CI, 92.6-94.1%); and 93.3%, 88.9% and 100% for LDG1, LDG2 and LDG3, respectively; BOS 91.7% (95% CI, 90-93%); and 100% for LDG1 and LDG2 and 75% for LDG3 (Figure 1 a,b,c and d).
One patient on NIL required DR for grade 3 liver toxicity, progressed to blast crisis after losing MR3 on 300mg daily and died post allo-SCT. One patient, who had achieved only CHR on IM, developed a T315I mutation on 50mg DAS second line while in MR3, having lost MR4, and was changed to ponatinib. One patient on DAS died of an unrelated brain tumour.
In each TKI cohort, 59/83 pts (71%) remained on LD IM, 51/75 (68%) pts on LD DAS, 35/72 (48.6%) pts on LD NIL and 30/32 (93.7%) pts on LD BOS. 55 pts stopped LD TKI while in sustained MR4 or greater (IM n=21/83 [25.3%], DAS n=12/75 [16%], NIL n=20/72 [27.8%], BOS n=2/30 [6.6%]) with a 2-year probability of TFR of 79.4% (95% CI, 78.3-80.5%) (compared to 50% at 2 years in EURO-SKI), with a median observation time of 28 months (5-83.7) in non-relapsing pts. TFR in the different cohorts were 85.7%, 62.3%, 80% and 100% for IM, DAS, NIL and BOS respectively.
Conclusion: For selected pts in ≥MR3 lowering the TKI dose can improve the tolerability of TKI therapy without impacting responses. The higher rate of TFR observed in our pts than in published stopping studies probably reflects cohorts of pts already shown to maintain deep responses on lower than standard doses of TKI, and mirrors the results of the UK NIHR Destiny study.
Apperley:Incyte: Honoraria, Speakers Bureau; BMS: Honoraria, Speakers Bureau; Pfizer: Honoraria, Speakers Bureau; Novartis: Honoraria, Research Funding, Speakers Bureau. Milojkovic:Incyte: Honoraria, Speakers Bureau; Novartis: Honoraria, Speakers Bureau; Pfizer: Honoraria, Speakers Bureau; BMS: Honoraria, Speakers Bureau.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal